|
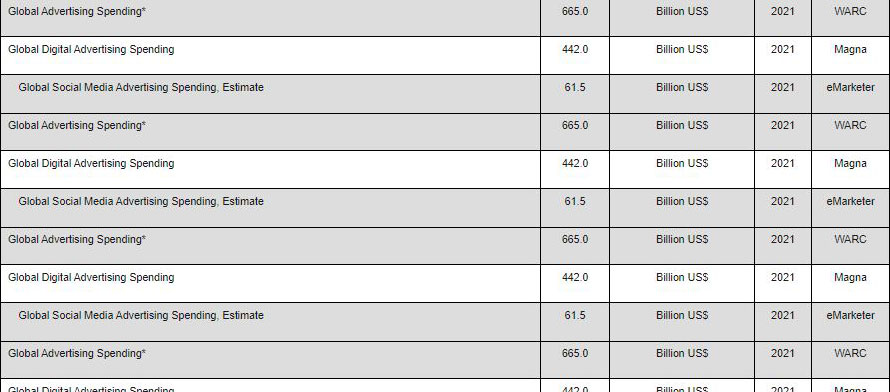
U.S. Pharmaceutical R&D Spending Versus the Number of New Molecular Entity (NME) Approvals: 1994-2020 |
|
|
|
|
|
* Beginning in 2004, these figures include new BLAs for therapeutic biologic products transferred from CBER to CDER. |
|
|
Notes: The FDA defines a New Molecular Entity (NME) as a medication containing an active substance that has never before been approved for marketing in any form in the U.S. Pharmaceutical R&D Spending includes expenditures inside and outside the U.S. by U.S.-owned PhRMA member companies and R&D conducted inside and outside the U.S. by the U.S. divisions of foreign-owned PhRMA member companies. R&D performed by the foreign divisions of foreign-owned PhRMA member companies is excluded. |
|
|
Source: Pharmaceutical Research and Manufacturers Association (PhRMA); U.S. Food and Drug Administration |
Become a Plunkett Research Online subscriber to gain unlimited access to our massive amount of vital industry data and market research.
- ✔ 100,000 Industry Statistics
- ✔ 500 Industries Analyzed for Market Size, Profits and Forecasts
- ✔ 150,000 Industry Executives Profiled
- ✔ 25,000 Companies Profiled, Ranked and Analyzed
- ✔ 1,000 Vital Trends Analyzed, totaling 1,000,000 words
A Representative List of Organizations that Have Used our Research and Products:


